Richard J. Glyn and Graham Pattison*
J. Med. Chem. 2021, 64, 14, 10246–10259
In this paper we use a molecular matched pairs approach to examine the effects of changing hydroxy or methoxy groups on aromatic rings for fluorine atoms. We showed that the hydrogen bond acceptor ability of the phenol is critical to the difference in log P, and polarisable / hydrogen-bonding groups elsewhere in the molecule are also important. We examied the effects on both simple aromatic rings and more complex drug-like molecules
G.R.A. Garratt; G. Pattison*
Synlett, 2020, 31, 1656-1662
This is s Synpacts article summarising our recent results, as well as those of other groups, in the synthesis of boron enolates by the substitution of esters with geminal bis(boron) compounds. Recent results from undergraduate student George Garratt towards the attempted sequential trapping of each boron atom in a bis(boron) enolate with a different electrophile are also included
H. Pinfold, G. Pattison, G. Costantini,
CrystEngComm, 2020, 22, 2425-2428
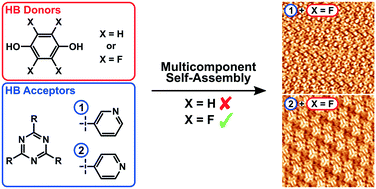
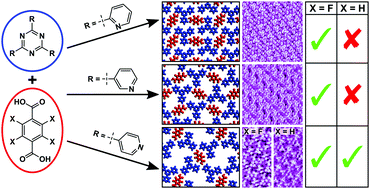
Collaborative research with the Costantini group (Warwick) on the 2D self-assembly of fluorinated phenols with hydrogen bond acceptors
Fluorinated Carboxylic Acids as Powerful Building Blocks for the Formation of Bimolecular Monolayers
H. Pinfold, C. Greenland, G. Pattison, G. Costantini
Chem. Commun. 2020, 56, 125-128
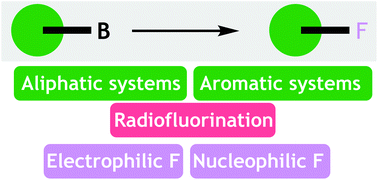
G. Pattison*
Org. Biomol. Chem. 2019, 17, 5651.
A review article on the fluorination of organoboron compounds, covering methods for the conversion of C-B to C-F bonds up to 2019. Methods for radiofluorination are also included. This article was invited as part of a special collection in fluorine chemistry to celebrate the 22nd International Symposium on Fluorine Chemistry, Oxford.
G. Pattison*
Eur. J. Org. Chem. 2018, 3520
This review article covers synthetic methods for the synthesis of difluorinated ketones. It was invited for a special issue on Fluorine Chemistry in Europe and was highlighted as a Cover Feature.
C.E. Iacono; T.C. Stephens; T.S. Rajan; G.Pattison*
J. Am. Chem. Soc. 2018, 140, 2036-2040
Our initial report on the synthesis of bis(boron) enolates by nucleophilic substitution of esters with geminal bis(boron) compounds. This strategy lets you predictably and selectively form two bonds with electrophiles adjacent to a carbonyl group without having to worry about traditional issues of enolate formation selectivity
G. Pattison*
Beilstein J. Org. Chem. 2017, 13, 2915–2921
A combined computational and experimental study. We observed that α-fluorinated ketones are in fact a little less reactive towards nucleophilic addition than α-chlorinated and brominated ketones. We attribute this to the reactive conformation, in which halogen lies at 90° to the carbonyl group, being less favourable in the fluorinated case.
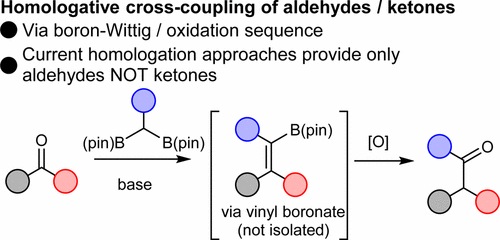
T.C. Stephens; G. Pattison*
Org. Lett. 2017, 19, 3498.
We have developed the first homologative coupling of aldehydes and ketones, a reaction which both extends a carbon chain by one carbon and introduces a new ketone substituent. This reaction proceeds via a boron-Wittig / oxidation sequence, through the addition of a geminal bis(boron) species to an aldehyde or ketone.
L.S. Dobson; G. Pattison*
Chem. Commun. 2016, 52, 11116.
This paper presents the Rh-catalysed addition of arylboronic acids to fluorinated ketones. We showed that fluorination was crucial to the activation of ketones for this reaction to proceed, and that a CF2H group was more activating than a CF3 group in this process.
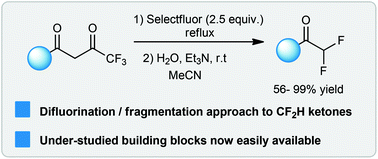
D.J Leng; C.M. Black; G. Pattison*
Org. Biomol. Chem. 2016, 14, 1531.
Here we report a synthesis of difluoromethyl (CF2H) ketones by a difluorination/fragmentation of fluorinated 1,3-dicarbonyl compounds. These simple ketones were surprisingly difficult to make previously. We showed the importance of water and base to this process
G. Pattison*
in ‘Green and Sustainable Medicinal Chemistry: Methods, Tools and Strategies for the 21st Century Pharmaceutical Industry, 2016, 203.
A book chapter describing a selection of modern appraches to doing halogenation in a way that is sustainable and minimizes environmental impact.
T.J Nash; G. Pattison*
Eur. J. Org. Chem. 2015, 3779.
This article described a method for the fluorination of 1,3-dicarbonyl compounds using a nucleophilic source of HF, mediated by I(III). The mechanism of this reaction was studied to give more information about the selectivity observed.








%20mediated%20fluorination.png)